Materials to be adapted:
displacement tank
density cubes (steel, wood, aluminum, copper, PVC)
100ml graduated cylinder
A displacement tank is a large plastic beaker with a spigot on the side. When an object is placed into the full tank, water will flow out the side to be collected in a beaker. The water is then measured in a graduated cylinder to determine the amount displaced by the object------volume displaced=volume of the object.
I did not want to try to rig up a displacement tank so I decided we'd just measure displacement by the use of a graduated cylinder------measure the water level in the graduated cylinder, place the object in the cylinder, measure the new water level to determine the volume of water displaced by the object.
Unfortunately we didn't have a graduated cylinder. Ooops. I used an old medicine dosing cup with milliliter markings to make a juice glass into a temporary graduated cylinder:
Rough measurements to be sure but the purpose of the lab was to introduce the concept of density.
Density cubes were to be used during the first portion of the lab. The cubes are of the same size but are made of different materials. Students then do not focus on size/shape of the objects, but rather on the behavior of the various materials. As much as I would have loved to use density cubes (see the Nasco catalog here), I couldn't justify the expense for a single lab. We then turned to regular-shaped objects found in the house-----a Jenga block, a magnet, a die, and a cube of brass Lab Assistant cut from a larger bar. The regular (either cubic or rectangular prism) shapes would enable Daisy to easily determine the corresponding volumes by length x width x height. I couldn't find a bar magnet, so I substituted the circular one and held a quick review of the volume of a cylinder.
For the first section with regular-shaped objects, she was to predict whether each object would sink or float. Then she found the mass of each on our balance (food scale, thankyouverymuch), used a ruler to determine the volumes, calculated the density, and then plopped each into our "graduated cylinder" to see if it would sink or float. She actually thought the die would be neutrally buoyant--would neither sink nor float--and was disappointed that it did sink.
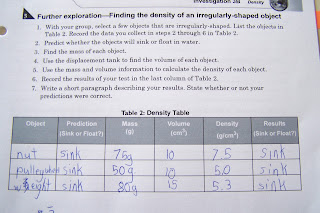
The next portion involved determining the density of irregular objects.
You can view the data table below. The volume of each was determined by the displacement method.
Carefully placing objects into the graduated cylinder:

It was a bit tricky removing some objects from the bottom of the graduated cylinder!
Thanks for reading about another fun, hands-on lab with CPO Earth Science!




OH! This will be an awesome resource!! I'm glad you're a compulsive-researcher! It'll help so many later on!
ReplyDelete